
Drug Development
Medicinal chemistry is integral to the drug development process, resulting in thousands of drug-like compounds synthesized yearly. Technologies like combinatorial chemistry and microwave-assisted organic synthesis have increased the speed of drug development, yet rapid purification and analytical characterization of these compounds remain a challenge.
Utilizing small sample quantities and reducing waste are key factors in cost minimization and accelerating synthesis/discover steps. Whether it is a biosimilar or larger biopharmaceutical drug or conventional small molecule synthesis, quick reaction monitoring is crucial to increase the yield (assay) and to identify and control product impurities.

Water determination, assay monitoring, and product impurity determination are important steps of drug development. Navigate the tabs below to gain some insight, access webinars, and other resources.
Water Determination
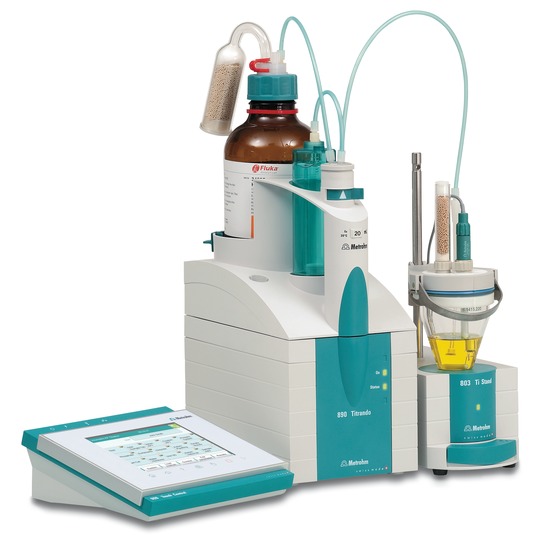 High-purity, water-free solvents are needed in demanding organic chemistry. Accurate moisture analysis requires looking at free and bound moisture from percent to ppm levels. Karl Fischer (KF) titration is the water-specific approach for ensuring accurate results. Properties of some samples can disrupt the KF reaction. However, Metrohm is experienced in helping you navigate these samples with tips for extraction, sample preparation, titration parameter adjustment and reagent selection.
High-purity, water-free solvents are needed in demanding organic chemistry. Accurate moisture analysis requires looking at free and bound moisture from percent to ppm levels. Karl Fischer (KF) titration is the water-specific approach for ensuring accurate results. Properties of some samples can disrupt the KF reaction. However, Metrohm is experienced in helping you navigate these samples with tips for extraction, sample preparation, titration parameter adjustment and reagent selection.
Assay Monitoring
 Many assay methods described in the U.S. and European Pharmacopoeias rely on the accuracy and simplicity of titration. Leveraging the flexibility of titration requires understanding how to optimize solvent selection or scale to a microtitration system.
Many assay methods described in the U.S. and European Pharmacopoeias rely on the accuracy and simplicity of titration. Leveraging the flexibility of titration requires understanding how to optimize solvent selection or scale to a microtitration system.
Product Impurity Determination
 Impurities in Pharmaceuticals mostly stem from synthesis and/or the starting materials used. Generally, their concentration is very low, and therefore analysis at trace levels is required. Metrohm offers the appropriate instruments and a broad range of applications that deal with impurity profiling – pharmacopeia-compliant, of course.
Impurities in Pharmaceuticals mostly stem from synthesis and/or the starting materials used. Generally, their concentration is very low, and therefore analysis at trace levels is required. Metrohm offers the appropriate instruments and a broad range of applications that deal with impurity profiling – pharmacopeia-compliant, of course.
Biosensors for Pharmacology
 Electrochemical biosensors are an effective tool for therapeutic response monitoring to characterize the interactions of the drug with the target receptor, cell, or other biological systems. There are a wide variety of electrode materials available from macro electrodes down to microelectrodes and ultramicroelectrodes. Microsensor arrays have also been used for rapid, complex sensing. Biosensor selectivity can be controlled by surface functionalization with cofactors, catalytic enzymes, or other targeted organic or inorganic sites; the applied potential can also dictate the sensor’s selectivity. A wealth of literature has reported the use of electroanalytical biosensors.
Electrochemical biosensors are an effective tool for therapeutic response monitoring to characterize the interactions of the drug with the target receptor, cell, or other biological systems. There are a wide variety of electrode materials available from macro electrodes down to microelectrodes and ultramicroelectrodes. Microsensor arrays have also been used for rapid, complex sensing. Biosensor selectivity can be controlled by surface functionalization with cofactors, catalytic enzymes, or other targeted organic or inorganic sites; the applied potential can also dictate the sensor’s selectivity. A wealth of literature has reported the use of electroanalytical biosensors.
 Biosensors have also been applied to the study of drug pathways and metabolism for therapeutic drug monitoring (TDM). The versatility of biosensor techniques, electrodes, and surface functionalization has led to diverse applications to in-vivo, in-vitro, imaging, or fundamental biochemical experiments. Electrochemical sensors give researchers the power to control the applied waveform, and this gives rise to interesting methodologies such as time-resolved, in-situ experiments.
Biosensors have also been applied to the study of drug pathways and metabolism for therapeutic drug monitoring (TDM). The versatility of biosensor techniques, electrodes, and surface functionalization has led to diverse applications to in-vivo, in-vitro, imaging, or fundamental biochemical experiments. Electrochemical sensors give researchers the power to control the applied waveform, and this gives rise to interesting methodologies such as time-resolved, in-situ experiments.
 The engine behind a novel biosensor is a potentiostat/galvanostat. This a versatile instrument that can apply a set potential (voltage) waveform and measure the resulting current response signal resulting from charge-accumulation or charge-transfer processes at the electrode-electrolyte interface. Conversely, a current waveform can be applied to drive a charge-transfer process while measuring the resulting potential response.
The engine behind a novel biosensor is a potentiostat/galvanostat. This a versatile instrument that can apply a set potential (voltage) waveform and measure the resulting current response signal resulting from charge-accumulation or charge-transfer processes at the electrode-electrolyte interface. Conversely, a current waveform can be applied to drive a charge-transfer process while measuring the resulting potential response.
There are various techniques used for biosensing that apply a constant-hold (chronoamoperomy or chronopotentiometry), sweep (linear or cyclic voltammetry), pulse (differential pulse, square-wave pulse, or others), or sequence of these waveforms. Electrochemical impedance spectroscopy (EIS), an alternating-current (AC) technique, has also been widely used to acquire in-depth information about the cell understudies such as binding events on a functionalized surface, charge-transfer resistance, multi-step reaction mechanisms, electrolyte resistance, and diffusion. Unlike source measure units, high-quality potentiostats/galvanostats have customizable capabilities such as EIS, low-current, or fast-scanning, and they have advanced software for implementing and these techniques and analyzing the data.